What is iLivTouch® ?
|
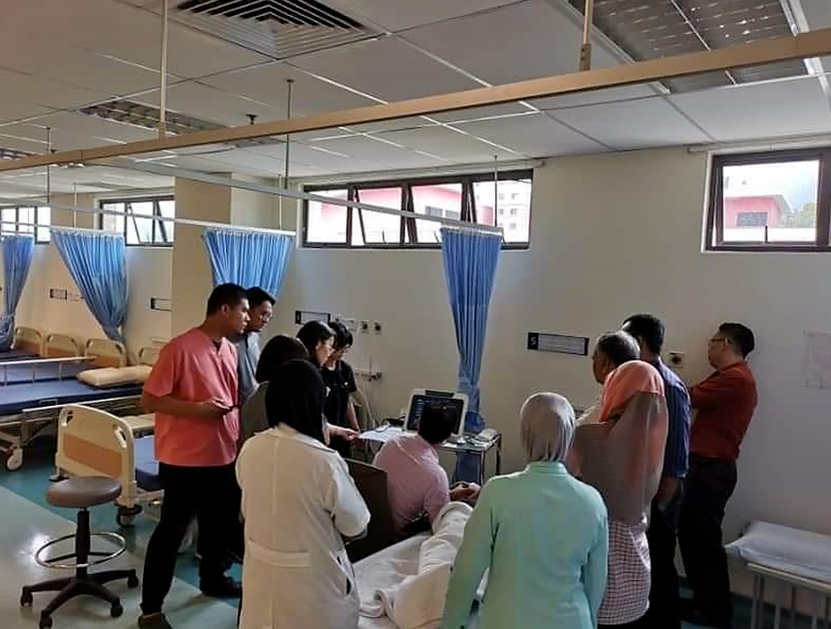
iLivTouch is a non-invasive device that provides the simultaneous assessment for liver fibrosis and steatosis via transient elastography (TE) technique. In recent years, TE has been widely adopted and highly recommended for clinical use by numerous international guidelines. Essentially, iLivTouch is useful for patients with liver diseases in detecting advanced liver diseases, monitoring the disease progression or therapeutic response, and facilitating the treatment decision.
|
How does iLivTouch® Work?
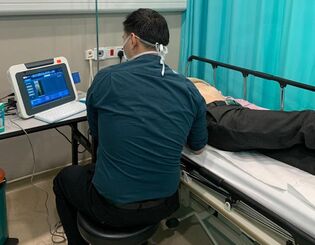
In TE technique, a small transducer on the tip of the ultrasound probe will transmit a low frequency shear wave (50Hz) toward the liver tissue. The region of interest measured amounts to a volume of 4cm^3, which is approximately 100 times larger than the sampling volume of liver biopsy.
|
TE device computes the liver stiffness measurement (LSM) by measuring the velocity of the shear wave as it propagates through the liver. The value is expressed in kilopascals (kPa), and a higher LSM reflects a higher degree of liver fibrosis.
|
|
iLivTouch also provides a quantitative measure of hepatic steatosis by determining the ultrasound signal attenuation rate. The measurement is expressed as ultrasound attenuation parameter (UAP) in the unit of decibel per meter (dB/m), and a higher number indicates more pronounced steatosis.
|
(UAP) Ultrasound Attenuation Parameter
|
Meet The Founders
How iLivTouch® is made possible.
About HISKY Medical
Wuxi Hisky Medical Technologies Co., Ltd. (HISKY) is a rapidly growing company specialized in developing, manufacturing, and marketing of innovative medical equipment. Founded in 2010, HISKY has been focusing on research application developments as well as the marketing of cutting-edge acoustic technology in the healthcare industry. They constantly thrive to add more and more innovative solutions for liver fibrosis and hepatic steatosis diagnosis, hence, the iLivTouch®. Their commitment and hard-work has allowed to further grow and extend their opportunities beyond their home market in China.
With the core technology of instantaneous elastic imaging, HISKY is committed to apply their knowledge of such technology to provide a non-invasive, fast, convenient and repeatable disease diagnosis and monitoring of liver and other organs for healthcare professionals, and truly realizing the "early diagnosis, early prevention, early treatment and early rehabilitation" of chronic liver diseases.
Operation Quick Guide
|
|
Operating our single dynamic probe iLivTouch® is swift, simple and accurate.
Check out the YouTube video on the left to learn more about: • Patient positioning during examination & scanning region • Probe grip method • User interface workflow • Performance measures of assessment results |
CertificationiLivTouch® has passed the certification of TUV SUD Group, EU official certification authority and obtained the CE certificate while bring certified with ISO13485. This allows our production system and products to officially be recognized by the EU. In addition, it has also been approved by the US FDA in 2018.
The Malaysian Medical Device Authority has also given its approval for its application and registration back in 2019. |
INTERESTED FOR A DEMO? HAVE QUESTIONS?
give us a ring, drop us an email or WhatsApp, and we'll get back to you ASAP
*only available for Malaysia, for individuals who are interested, please contact the respective regional distributor.
*only available for Malaysia, for individuals who are interested, please contact the respective regional distributor.
|
|
phone |
+60 12-212 1920 Ms. June
+60 19-664 8984 Mr. Owe +60 12-569 7992 Ms. Jessie +60 12-587 3992 Mr. Gan |
address |
No 39 - 1,
Jalan Kuchai Maju 7, Off Jalan Kuchai Lama, Kuchai Entrepreneur's Park, 58200 Kuala Lumpur. |